Sunday, November 27, 2022
CT screening for early lung cancer leads to long-term survival

Axial CT images of pulmonary nodules.
(A) Malignant nodule. (B) Benign nodule.
Image credit – RSNA
CANCER DIGEST – Nov. 27, 2022 – A new study shows that early detection of lung cancer with CT scanning dramatically increases long-term survival.
The study led by Claudia Henschke, PhD, MD of the Icahn School of Medicine at Mount Sinai in New York, follows 87,000 participants at 80 cancer centers who have been diagnosed with early stage lung cancer. The results were presented at the annual meeting of the Radiological Society of North America in Chicago.
 |
| Axial CT images of pulmonary nodules. (A) Malignant nodule. (B) Benign nodule. Image credit – RSNA |
The study led by Claudia Henschke, PhD, MD of the Icahn School of Medicine at Mount Sinai in New York, follows 87,000 participants at 80 cancer centers who have been diagnosed with early stage lung cancer. The results were presented at the annual meeting of the Radiological Society of North America in Chicago.
Saturday, October 2, 2021
Key to treating lung cancer in never-smokers identified
An image of a lung tumor in a patient who never smoked
Image credit Washington University
CANCER DIGEST – Oct. 2, 2021 – Lung cancer in people who have never smoked has long been a mystery to researchers, and although they still are working to understand the origin of these cancer, researchers at Washington University in St. Louis have found that these tumors are treatable with existing therapies.
 |
| An image of a lung tumor in a patient who never smoked Image credit Washington University |
CANCER DIGEST – Oct. 2, 2021 – Lung cancer in people who have never smoked has long been a mystery to researchers, and although they still are working to understand the origin of these cancer, researchers at Washington University in St. Louis have found that these tumors are treatable with existing therapies.
Saturday, March 13, 2021
Review finds benefits of lung cancer screening outweigh harms
CANCER DIGEST – March 13, 2021 – Lung cancer screening with computed tomography or CT scans does detect early lung cancers and reduce deaths due to lung cancer, but it is not without some adverse consequences, according to a comprehensive review by University of North Carolina researchers.The review is published in JAMA on March 9, 2021.
"Applying screening tests to a population without symptoms of disease can certainly benefit some people but also has the potential for some harms," said lead author Daniel Jonas, MD, MPH, who conducted most of this research while he was a professor at the UNC School of Medicine. "In the case of lung cancer screening, we now have more certainty that some individuals will benefit, with some lung cancer deaths prevented, and we also know others will be harmed."
CANCER DIGEST – March 13, 2021 – Lung cancer screening with computed tomography or CT scans does detect early lung cancers and reduce deaths due to lung cancer, but it is not without some adverse consequences, according to a comprehensive review by University of North Carolina researchers.The review is published in JAMA on March 9, 2021.
"Applying screening tests to a population without symptoms of disease can certainly benefit some people but also has the potential for some harms," said lead author Daniel Jonas, MD, MPH, who conducted most of this research while he was a professor at the UNC School of Medicine. "In the case of lung cancer screening, we now have more certainty that some individuals will benefit, with some lung cancer deaths prevented, and we also know others will be harmed."
"Applying screening tests to a population without symptoms of disease can certainly benefit some people but also has the potential for some harms," said lead author Daniel Jonas, MD, MPH, who conducted most of this research while he was a professor at the UNC School of Medicine. "In the case of lung cancer screening, we now have more certainty that some individuals will benefit, with some lung cancer deaths prevented, and we also know others will be harmed."
Saturday, January 2, 2021
Light smokers likely just as addicted as heavy smokers

Photo courtesy of Penn State News
CANCER DIGEST – Jan. 2, 2021 –People who smoke as few as one to four cigarettes a day are addicted to nicotine and find quitting altogether to be very difficult without assistance, a new study shows. The findings appear in the Dec. 22, 2020 American Journal of Preventive Medicine.
Such smokers considered "light smokers" have correctly perceived that their habit is less harmful than heavy smoking, but it still carries significant medical health risks. Medical providers have tended to consider "light smokers" as not addicted and, therefor not in need of treatment, however this study suggests many of these patients are addicted and will not successfully quit without treatment.
 |
| Photo courtesy of Penn State News |
Such smokers considered "light smokers" have correctly perceived that their habit is less harmful than heavy smoking, but it still carries significant medical health risks. Medical providers have tended to consider "light smokers" as not addicted and, therefor not in need of treatment, however this study suggests many of these patients are addicted and will not successfully quit without treatment.
Saturday, April 27, 2019
Lung cancer risks in never smokers under recognized

Air pollution is a risk factor for lung cancer that is
overlooked – Image Wikipedia Creative
Commons copyright.
CANCER DIGEST – April 27, 2019 – About 30,000 Americans who have never smoked die of lung cancer each year, according to the American Cancer Society.
Now a group of British respiratory therapists and public health experts are calling for greater recognition of lung cancer in never-smokers.
 |
| Air pollution is a risk factor for lung cancer that is overlooked – Image Wikipedia Creative Commons copyright. |
Now a group of British respiratory therapists and public health experts are calling for greater recognition of lung cancer in never-smokers.
Saturday, April 14, 2018
Double-drug strategy blocks escape route for most lung cancers

The tumors in the top three rows above were treated with
current drugs, the bottom row tumors were treated with the
new combination. Image courtesy of UT Southwestern
CANCER DIGEST – April 14, 2018 – A combination two already FDA approved drugs appear to be effective in treating non-small lung cancer, at least in mice models that had human-derived tumors, researchers say. Because the two drugs are already approved, the researchers hope to have clinical trial in human approved within a year.
The two drugs include a class of drugs called EGFR inhibitors such as Erbitux®, Tarceva® and Iressa® and the class of TNF inhibitors such as Humira®, Enbrel® and Remicade®.
 |
| The tumors in the top three rows above were treated with current drugs, the bottom row tumors were treated with the new combination. Image courtesy of UT Southwestern |
The two drugs include a class of drugs called EGFR inhibitors such as Erbitux®, Tarceva® and Iressa® and the class of TNF inhibitors such as Humira®, Enbrel® and Remicade®.
Sunday, April 8, 2018
New combination shows promise in advanced lung cancer
CANCER DIGEST – April 8, 2018 – A new combination therapy of using an immunotherapy drug, with a new and powerful immune stimulation drug, shows promise in patients whose advanced lung cancer has become resistant to other therapies, preliminary results of a clinical trial shows.
The study is the first time the immune stimulant has been combined with one of the new class of drugs, called checkpoint drugs such as nivolumab (OPDIVO®) and pembrolizumab (Keytruda) according to study leaders Drs. John Wrangle, and Mark Rubinstein, PhD., of the Hollings Cancer Center at the Medical University of South Carolina.
CANCER DIGEST – April 8, 2018 – A new combination therapy of using an immunotherapy drug, with a new and powerful immune stimulation drug, shows promise in patients whose advanced lung cancer has become resistant to other therapies, preliminary results of a clinical trial shows.
The study is the first time the immune stimulant has been combined with one of the new class of drugs, called checkpoint drugs such as nivolumab (OPDIVO®) and pembrolizumab (Keytruda) according to study leaders Drs. John Wrangle, and Mark Rubinstein, PhD., of the Hollings Cancer Center at the Medical University of South Carolina.
The study is the first time the immune stimulant has been combined with one of the new class of drugs, called checkpoint drugs such as nivolumab (OPDIVO®) and pembrolizumab (Keytruda) according to study leaders Drs. John Wrangle, and Mark Rubinstein, PhD., of the Hollings Cancer Center at the Medical University of South Carolina.
Saturday, October 14, 2017
Newly approved breast cancer drug may work for lung cancer
 |
| This image shows autophagic vesicles containing mutant K-Ras formed in the membrane of human pancreatic cancer cells after exposure to neratinib – Image courtesy VCU. |
The drug neratinib was designed to inhibit enzymes produced by two other genes, EGFR and HER2, which make enzymes that regulate cancer cell growth and resistance to chemotherapy.
0 commentsSaturday, July 29, 2017
Agent makes tumors glow for surgeons
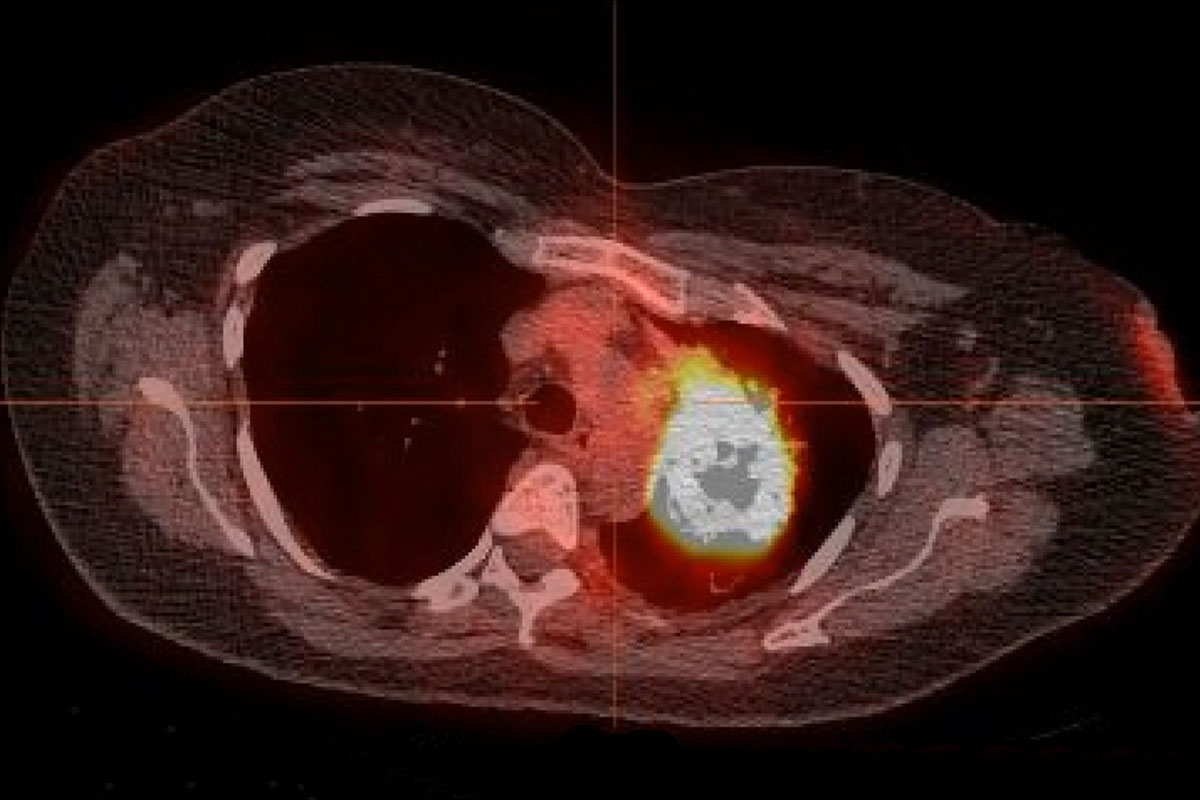
CANCER DIGEST – July 29, 2017 – Using a combination of imaging technologies and an agent that makes tumors glow, surgeons identified and removed more cancerous nodules from lung cancer patients than they would have using preoperative PET scans alone, researchers report.
The study by researchers at the Abramson Cancer Center of the University of Pennsylvania combined pre-operative PET scans with a molecular imaging technique during surgery using a contrast dye that is taken up by tumors and makes them glow. The glowing tumors are then easier for surgeons to see and remove.
CANCER DIGEST – July 29, 2017 – Using a combination of imaging technologies and an agent that makes tumors glow, surgeons identified and removed more cancerous nodules from lung cancer patients than they would have using preoperative PET scans alone, researchers report.
The study by researchers at the Abramson Cancer Center of the University of Pennsylvania combined pre-operative PET scans with a molecular imaging technique during surgery using a contrast dye that is taken up by tumors and makes them glow. The glowing tumors are then easier for surgeons to see and remove.
Saturday, July 1, 2017
Focused lung cancer screening cost-effective
CANCER DIGEST – June 30, 2017 – Focusing on high-risk people and expanding the scope to other tobacco-related diseases make lung-cancer screening programs cost-effective, researchers say.
In a cost-of-care-study Canadian researchers found that focusing on high-risk people could reduce the number of people who need to be screened by more than 80 percent, and calculated the cost of screening to be $20,724 (in 2015 Canadian dollars) per year of life saved; this means
CANCER DIGEST – June 30, 2017 – Focusing on high-risk people and expanding the scope to other tobacco-related diseases make lung-cancer screening programs cost-effective, researchers say.
In a cost-of-care-study Canadian researchers found that focusing on high-risk people could reduce the number of people who need to be screened by more than 80 percent, and calculated the cost of screening to be $20,724 (in 2015 Canadian dollars) per year of life saved; this means
In a cost-of-care-study Canadian researchers found that focusing on high-risk people could reduce the number of people who need to be screened by more than 80 percent, and calculated the cost of screening to be $20,724 (in 2015 Canadian dollars) per year of life saved; this means
Monday, October 17, 2016
Blood tests can cut time between diagnosis and treatment
CANCER DIGEST – Oct. 17, 2016 – Patients at high risk for lung cancer who have a blood test to identify certain genetic mutations known to play a role in development different types of lung cancer can sharply reduce the time needed to decide on a treatment and start treatment once they are diagnosed with lung cancer, a new study suggests.
Early results of the study will be presented at the 2016 annual meeting of the American College of Chest Physicians, in Los Angeles Oct. 26, ahead of publication in the journal CHEST.
CANCER DIGEST – Oct. 17, 2016 – Patients at high risk for lung cancer who have a blood test to identify certain genetic mutations known to play a role in development different types of lung cancer can sharply reduce the time needed to decide on a treatment and start treatment once they are diagnosed with lung cancer, a new study suggests.
Early results of the study will be presented at the 2016 annual meeting of the American College of Chest Physicians, in Los Angeles Oct. 26, ahead of publication in the journal CHEST.
Monday, December 14, 2015
FDA approves new drug for sub-type of non-small cell lung cancer
CANCER DIGEST – Dec. 14, 2015 – The U.S. Food and Drug Administration approved Alecensa (alectinib) to treat people with specific form of non-small cell lung cancer.
The Dec. 11 approval was for non-small cell lung tumors that have a mutation in the anaplastic lymphoma kinase (ALK) gene, which is present in several types of cancer in addition to about 5 percent of non-small cell lung cancers. The approval is for treatment in patients whose disease has worsened after treatment with a drug called Xalkori (crizotinib), or who could not tolerate it.
CANCER DIGEST – Dec. 14, 2015 – The U.S. Food and Drug Administration approved Alecensa (alectinib) to treat people with specific form of non-small cell lung cancer.
The Dec. 11 approval was for non-small cell lung tumors that have a mutation in the anaplastic lymphoma kinase (ALK) gene, which is present in several types of cancer in addition to about 5 percent of non-small cell lung cancers. The approval is for treatment in patients whose disease has worsened after treatment with a drug called Xalkori (crizotinib), or who could not tolerate it.
Thursday, September 10, 2015
New targeted therapy improves lung cancer survival

Image courtesy NCI
CANCER DIGEST – Sept. 10, 2015 – The world’s largest clinical trial comparing two targeted therapies for a form of advanced non-small cell lung cancer has found that a newer medication called afatinib decreased the risk of cancer progression and the risk of death by 19 percent compared to an older therapy called erlotinib.
The type of non-small cell cancer studied is called squamous cell carcinoma of the lung, and begins in the tissue that lines the air passages in the lungs, most often located in the larger airways that join the lungs to the trachea or windpipe.
| Image courtesy NCI |
CANCER DIGEST – Sept. 10, 2015 – The world’s largest clinical trial comparing two targeted therapies for a form of advanced non-small cell lung cancer has found that a newer medication called afatinib decreased the risk of cancer progression and the risk of death by 19 percent compared to an older therapy called erlotinib.
The type of non-small cell cancer studied is called squamous cell carcinoma of the lung, and begins in the tissue that lines the air passages in the lungs, most often located in the larger airways that join the lungs to the trachea or windpipe.
Circulating tumor DNA can be used to tailor treatment
CANCER DIGEST – April 17, 2015 – Cancer DNA circulating in the bloodstream of lung cancer patients can provide doctors with vital mutation information that can help optimize treatment when tumor tissue is not available, an international group of researchers has reported at the European Lung Cancer Conference (ELCC) in Geneva, Switzerland.
The results have important implications for the use of cancer therapies that target specific cancer mutations, explains Dr Martin Reck from the Department of Thoracic Oncology at Lung Clinic Grosshansdorf, Germany, who presented the findings at the conference.
CANCER DIGEST – April 17, 2015 – Cancer DNA circulating in the bloodstream of lung cancer patients can provide doctors with vital mutation information that can help optimize treatment when tumor tissue is not available, an international group of researchers has reported at the European Lung Cancer Conference (ELCC) in Geneva, Switzerland.
The results have important implications for the use of cancer therapies that target specific cancer mutations, explains Dr Martin Reck from the Department of Thoracic Oncology at Lung Clinic Grosshansdorf, Germany, who presented the findings at the conference.
Thursday, March 26, 2015
Men’s midlife fitness linked to lower risk of cancer and death
CANCER DIGEST – Mar. 26, 2015 – Men who exercise and stay fit, especially in midlife, could be lowering their risk of lung cancer and colorectal cancer, but not prostate cancer, researchers say. Before you say two out of three isn’t bad, while fitness didn’t protect against getting prostate cancer fit men appear to be less likely to die of the disease.
Led by Dr. Susan Lakoski of the University of Vermont, Burlington, the researchers looked at Medicare data from 1999 to 2009 for a link between midlife cardiorespiratory fitness and cancer and survival following a cancer diagnosis at the Medicare age of 65 or older.
CANCER DIGEST – Mar. 26, 2015 – Men who exercise and stay fit, especially in midlife, could be lowering their risk of lung cancer and colorectal cancer, but not prostate cancer, researchers say. Before you say two out of three isn’t bad, while fitness didn’t protect against getting prostate cancer fit men appear to be less likely to die of the disease.
Led by Dr. Susan Lakoski of the University of Vermont, Burlington, the researchers looked at Medicare data from 1999 to 2009 for a link between midlife cardiorespiratory fitness and cancer and survival following a cancer diagnosis at the Medicare age of 65 or older.
Thursday, February 5, 2015
Low-dose CT screening for lung cancer to be covered by Medicare

Licensed use - copyright Sebastian Kaulitzki
CANCER DIGEST – Feb. 5, 2015 – Lung cancer screening using low-dose CT scans will be covered by Medicare, which is good news for many older Americans who are at high risk for lung cancer.
The Centers for Medicare & Medicaid Services (CMS) announced its final coverage determination today. Medicare will now cover lung cancer screening with low-dose CT scans once per year for Medicare beneficiaries who meet all the following criteria:
 |
| Licensed use - copyright Sebastian Kaulitzki |
CANCER DIGEST – Feb. 5, 2015 – Lung cancer screening using low-dose CT scans will be covered by Medicare, which is good news for many older Americans who are at high risk for lung cancer.
The Centers for Medicare & Medicaid Services (CMS) announced its final coverage determination today. Medicare will now cover lung cancer screening with low-dose CT scans once per year for Medicare beneficiaries who meet all the following criteria:
Saturday, December 13, 2014
Saturday, December 13, 2014
FDA expands approval of Cyramza to non-small cell lung cancer
CANCER DIGEST – Dec. 13, 2014 – The FDA today expanded the approved use of Cyramza® (ramucirumab) to treat patients with metastatic non-small cell lung cancer (NSCLC).
Marketed by Eli Lily, Cyramza works by blocking the blood supply that fuels tumor growth. The drug is intended for NSCLC patients whose tumors have grown (progressed) during or following treatment with platinum-based chemotherapy, and it is to be used in combination with docetaxel, another type of chemotherapy.
Marketed by Eli Lily, Cyramza works by blocking the blood supply that fuels tumor growth. The drug is intended for NSCLC patients whose tumors have grown (progressed) during or following treatment with platinum-based chemotherapy, and it is to be used in combination with docetaxel, another type of chemotherapy.
Monday, July 21, 2014
Advances in chemo have lengthened non-small cell lung cancer survival

YouTube courtesy The Oncology Channel spon-
sored by Boehringer Ingelheim
CANCER DIGEST – July 21, 2014 – Patients with advanced non-small cell lung cancer survived significantly longer when treated with second and third line chemotherapy compared to patients who did not receive any chemotherapy, a new analysis of patient survival shows.
The researchers from the British Columbia Cancer Agency, Vancouver, Canada analyzed
 |
| YouTube courtesy The Oncology Channel spon- sored by Boehringer Ingelheim |
The researchers from the British Columbia Cancer Agency, Vancouver, Canada analyzed
Wednesday, May 14, 2014
Question about lung cancer screening decision answered
| Joshua Roth, PhD, MHA |
Labels: CT screening, Lung cancer, Medicare Advisory Panel
Thursday, May 1, 2014
Medicare panel shocks lung cancer experts by CT screening decision
 |
| YouTube comment on USPTF from MD Anderson – Aug. 2, 2013 |
Tuesday, April 29, 2014
FDA approves Zykadia for late-stage lung cancer
CANCER DIGEST – April 29, 2014 – The U.S. FDA today granted accelerated approval to Zykadia (ceritinib) for patients with a certain type of late-stage (metastatic) non-small cell lung cancer (NSCLC).
 The approval covers patients with tumors that overexpress anaplastic lymphoma kinase (ALK) and have progressed during or after treatment with crizotinib (Xalkori). Zykadia was shown to be highly active in these so called, ALK-rearranged non-small cell lung cancers. Its safety and effectiveness were established in a clinical trial of 163 participants with metastatic ALK-positive NSCLC. All participants were treated with Zykadia. Results showed that about half of the participants had their tumors shrink, and this effect lasted an average of about seven months. Although NSCLC accounts for about 85 percent of lung cancer, the ALK-positive subset makes up 2 percent to 7 percent of NSCLC.
The approval covers patients with tumors that overexpress anaplastic lymphoma kinase (ALK) and have progressed during or after treatment with crizotinib (Xalkori). Zykadia was shown to be highly active in these so called, ALK-rearranged non-small cell lung cancers. Its safety and effectiveness were established in a clinical trial of 163 participants with metastatic ALK-positive NSCLC. All participants were treated with Zykadia. Results showed that about half of the participants had their tumors shrink, and this effect lasted an average of about seven months. Although NSCLC accounts for about 85 percent of lung cancer, the ALK-positive subset makes up 2 percent to 7 percent of NSCLC. Wednesday, April 2, 2014
Lung cancer vaccine a dry hole for GlaxoSmithKline
REUTERS – April 2, 2014 – British drug maker GlaxoSmithKline announced that it has stopped its clinical trial of a immunotherapy vaccine for non-small cell lung cancer. The study comparing MAGE-A3 to standard therapy failed to show a significant benefit to people with the disease. The researchers are continuing to look for an effect of the drug on a subgroup of patients with a particular genetic make-up. The drug had previously failed to provide improvement in a clinical trial of melanoma patients.
Wednesday, March 26, 2014
Drug resistant lung cancer responds to new drug
HEALTH DAY – Mar. 26, 2014 – An early trial of an experimental lung cancer drug halted or shrank tumors in 58 percent of the 59 people treated. This response rate indicates the drug is highly active in patients with a specific type of advanced non-small cell lung cancer called, ALK-rearranged NSCLC. The result is encouraging for the drug’s maker and trial sponsor, Novartis, but it is not an indication of effectiveness. The objective of the trial is to determine the safety of the oral drug, ceritinib, and the optimal dose needed for further trials. Some of the patients in the study had previously been treated with another ALK-targeted drug called, crizotinib. The early results were published online today in the New England Journal of Medicine.
Smokers still unable to quit using e-cigarettes
MEDPAGE TODAY – Mar. 24, 2014 – An analysis of 949 smokers in a nationally representative panel followed from 2011 through 2012 showed that e-cigarette use made little difference in helping smokers quit or even smoke fewer tobacco cigarettes. The analysis was based on data from a online market research firm Knowledge Networks (now GfK). The study was published in theJAMA Internal Medicine. The results add to similar results of earlier studies.
Read more …
Federal task force gives thumbs up for CT screens for lung cancer
July 29, 2013 – ROCKVILLE, MD – Smokers got a double boost today in heading off their risk of dying from lung cancer. The U.S. Preventive Services Task Force (USPSTF), a panel of experts who examine the value of a variety of screening methods and the guidelines for using them, gave a thumbs up to using a yearly CT scan to screen for lung cancers.May 10, 2010 – ATLANTA (Cancer Digest) – Cost of cancer care doubled? So have other healthcare costs
Tuesday, May 4, 2010 – GENEVA (Cancer Digest) – Studies show frequent dose radiotherapy boosts survival









