 |
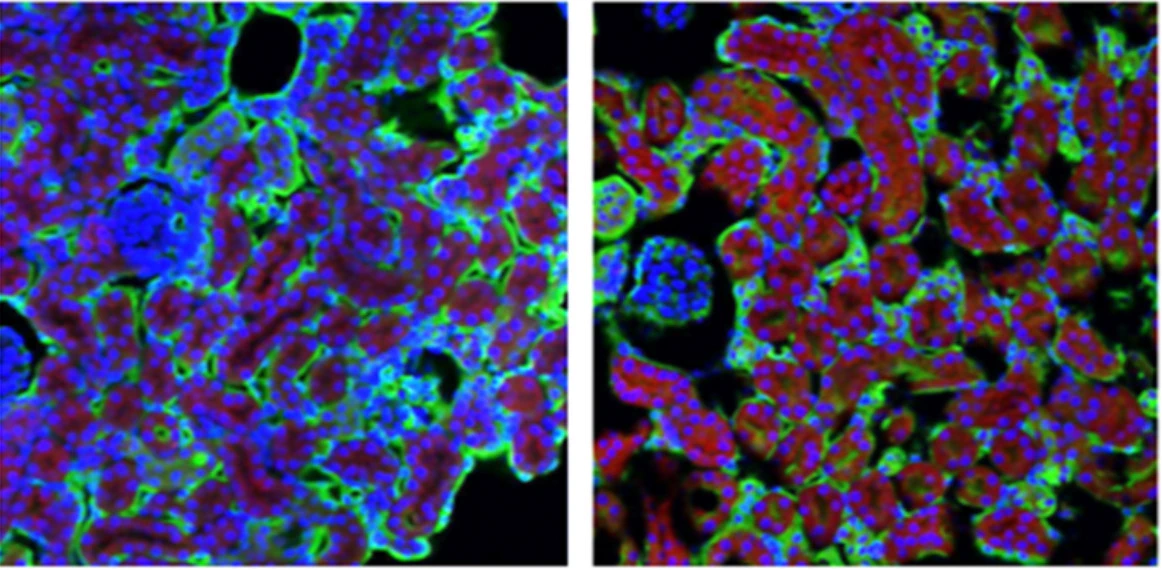
| Left shows mouse kidney cells and the right shows these same kidney cells after cisplatin treatment revealing they are full of adenosine (red). Image credit: Geoffroy Laumet |
The international research study conducted in mice was published in The Journal of Clinical Investigation published online Nov. 15, 2022. It showed that the drug istradefylline can reduce the side effects of cisplatin while preserving its cancer-fighting properties.
Cisplatin has been a mainstay of cancer chemotherapy for nearly 60 years. It is used to treat ovarian, lung, bladder, stomach and head and neck cancers. The side effects, however, can cause severe pain in the hands and feet, kidney failure, nausea and vomiting. Patients need to undergo weekly blood tests to monitor for kidney damage while on cisplatin.
Now with the findings of the current study led by Geoffroy Laumet at Michigan State University as part of four international teams at the University of Lille, the University of Strasbourg and the Pasteur Institute in France, and the University of Coimbra in Portugal, it appears that istradefylline might be used to substantially reduce those adverse side effects.
“The exact interaction between istradefylline and cisplatin remains to be determined but we do know that tumor cells and cells that are stressed by the toxicity of cisplatin will release a lot of adenosines,” Laumet said in a press release. “Istradefylline blocks the effects of adenosine.”
In the study, the researchers observed that the addition of istrdefylline increased production of a protein called A2AR, which appeared to alleviate the cisplatin-induced kidney toxicity without impacting the effectiveness of the tumor killing properties of cisplatin. It also significantly reduces kidney inflammation.
The researchers concluded that because istradefylline has already been proven safe in humans, a clinical trial in humans should be launched soon.
Sources: Michigan State University press release and The Journal of Clinical Investigation


No comments:
Post a Comment