Saturday, December 17, 2022
Adding a personalized vaccine to immunotherapy reduces recurrence in melanoma
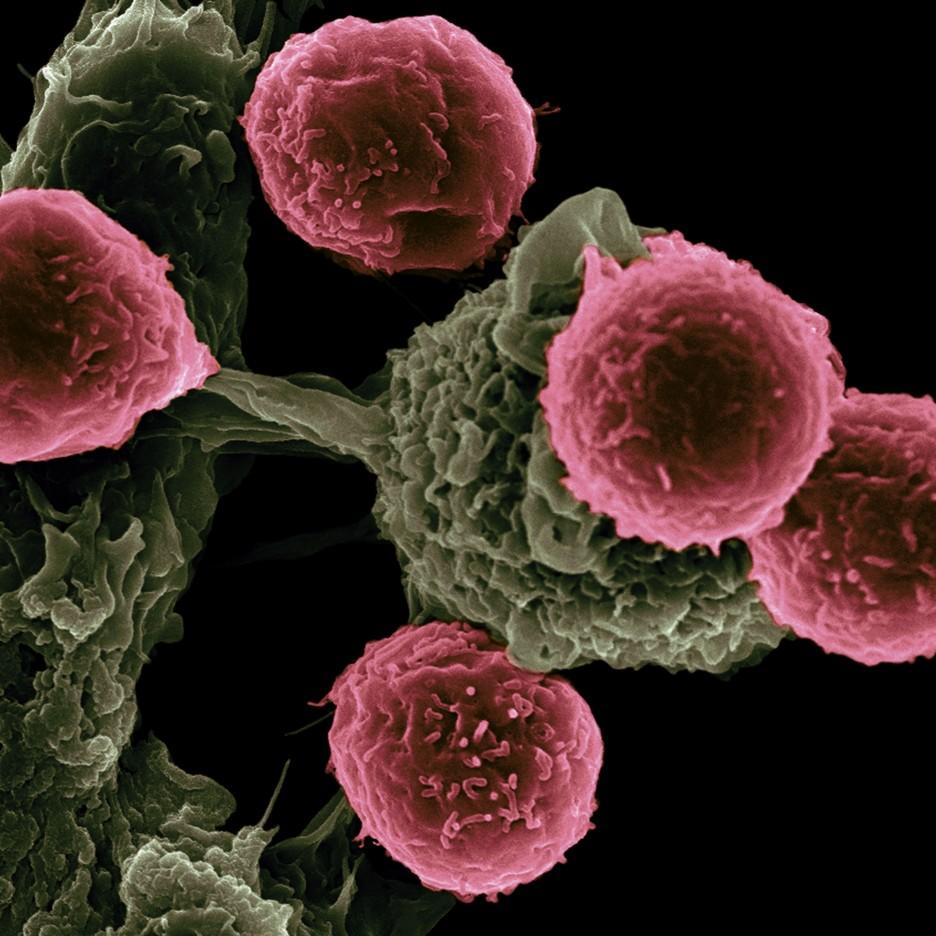
Treatment vaccines can help the immune system learn to
recognize and react to antigens and destroy cancer cells
that contain them. Credit: NCI and Victor Segura Ibarra and Rita Serda
CANCER DIGEST– Dec. 17, 2022 – Early results of a preliminary clinical trial shows that a combination therapy for stage 3/4 melanoma that has spread to lymph tissues in the body reduced recurrence and death by 44 percent.
 |
Treatment vaccines can help the immune system learn to recognize and react to antigens and destroy cancer cells that contain them. Credit: NCI and Victor Segura Ibarra and Rita Serda |
CANCER DIGEST– Dec. 17, 2022 – Early results of a preliminary clinical trial shows that a combination therapy for stage 3/4 melanoma that has spread to lymph tissues in the body reduced recurrence and death by 44 percent.
Sunday, October 2, 2022
Immunotherapy before targeted therapy boosts melanoma survival
Saturday, May 7, 2022
Handheld device accurately scans for skin cancer

Image of cancer from millimeter-wave device courtesy Stevens Institute of Technology
CANCER DIGEST – May 7, 2022 – When a crewmember of the Starship Enterprise became ill, Dr. McCoy would wave a wand-like device over the patient’s body and read the diagnosis. Now, researchers have developed a similar device that can detect skin cancer.
 |
Image of cancer from millimeter-wave device courtesy Stevens Institute of Technology |
CANCER DIGEST – May 7, 2022 – When a crewmember of the Starship Enterprise became ill, Dr. McCoy would wave a wand-like device over the patient’s body and read the diagnosis. Now, researchers have developed a similar device that can detect skin cancer.
Saturday, March 26, 2022
FDA approves new immunotherapy treatment for advanced melanoma

Photo credit – Oregon Health Sciences University
Fritz Liedtke
CANCER DIGEST – March 25, 2022 – There’s new hope for patients with an aggressive form of skin cancer that can’t be treated with surgery.
The FDA approved a new treatment regimen that combines two immunotherapy drugs that significantly extends progression-free survival.
The FDA approval follows the results of the RELATIVITY-047 clinical trial that involved 714 patients with advance, previously untreated melanoma.
Patients were randomly assigned to receive the combination therapy of relatlimab and nivolumab or nivolumab alone. Results of the trial were published in The New England Journal of Medicine on Jan. 6, 2022.
At one year after starting the trial, 48 percent of those in the combination therapy survived with the cancer not processing, called progression-free survival. That compared to 37 percent of the nivolumab group surviving that long without cancer progression.
 |
| Photo credit – Oregon Health Sciences University Fritz Liedtke |
CANCER DIGEST – March 25, 2022 – There’s new hope for patients with an aggressive form of skin cancer that can’t be treated with surgery.
The FDA approved a new treatment regimen that combines two immunotherapy drugs that significantly extends progression-free survival.
The FDA approval follows the results of the RELATIVITY-047 clinical trial that involved 714 patients with advance, previously untreated melanoma.
The FDA approval follows the results of the RELATIVITY-047 clinical trial that involved 714 patients with advance, previously untreated melanoma.
Patients were randomly assigned to receive the combination therapy of relatlimab and nivolumab or nivolumab alone. Results of the trial were published in The New England Journal of Medicine on Jan. 6, 2022.
At one year after starting the trial, 48 percent of those in the combination therapy survived with the cancer not processing, called progression-free survival. That compared to 37 percent of the nivolumab group surviving that long without cancer progression.
At one year after starting the trial, 48 percent of those in the combination therapy survived with the cancer not processing, called progression-free survival. That compared to 37 percent of the nivolumab group surviving that long without cancer progression.
Saturday, January 8, 2022
High fiber diet may boost effects of ICB therapy for melanoma patients
Saturday, June 19, 2021
COVID vaccine maker BioNTech launches phase 2 cancer vaccine trial
CANCER DIGEST – June 19, 2021 – BioNTech, the maker of the Pfizer COVID-19 vaccine, has turned its mRNA-based vaccine technology to cancer and has launched a phase 2 clinical trial to treat patients with relapsed melanoma that can’t be treated surgically.
The first dose of the BNT111 vaccine was given in combination with cemiplimab (Libtayo), a monoclonal antibody drug, in patients with melanoma that has not responded to other treatments. The clinical trial will enroll 120 patients to evaluate the effects of the combination treatment as well as the vaccine and Libtayo drug alone.
CANCER DIGEST – June 19, 2021 – BioNTech, the maker of the Pfizer COVID-19 vaccine, has turned its mRNA-based vaccine technology to cancer and has launched a phase 2 clinical trial to treat patients with relapsed melanoma that can’t be treated surgically.
The first dose of the BNT111 vaccine was given in combination with cemiplimab (Libtayo), a monoclonal antibody drug, in patients with melanoma that has not responded to other treatments. The clinical trial will enroll 120 patients to evaluate the effects of the combination treatment as well as the vaccine and Libtayo drug alone.
The first dose of the BNT111 vaccine was given in combination with cemiplimab (Libtayo), a monoclonal antibody drug, in patients with melanoma that has not responded to other treatments. The clinical trial will enroll 120 patients to evaluate the effects of the combination treatment as well as the vaccine and Libtayo drug alone.
Sunday, April 18, 2021
Movement disorder drug may prevent skin cancer recurrence
CANCER DIGEST – April 18, 2021 – A drug already approved for treatment of Parkinson’s and other neurotransmitter disorders may be effective in reducing skin cancer recurrence, say researchers at Ohio State University Comprehensive Cancer Center. Their findings appear in the April 12, journal Cancer Prevention Research.
CANCER DIGEST – April 18, 2021 – A drug already approved for treatment of Parkinson’s and other neurotransmitter disorders may be effective in reducing skin cancer recurrence, say researchers at Ohio State University Comprehensive Cancer Center. Their findings appear in the April 12, journal Cancer Prevention Research.
Saturday, January 23, 2021
A personalized cancer vaccine provides durable protection against melanoma
.jpg)
Patrick Ott, MD, PhD and Cathy Wu, MD
photo credit– Dana Farber
CANCER DIGEST – Jan. 23, 2021 – Melanoma patients who received a therapeutic vaccine tailored to their cancer show continued immune response to the cancer four years after treatment, a new study shows.
The small study involved eight patients who had undergone surgery for advanced melanoma but were considered at high risk of recurrence. Each received injections of NeoVax, a median of 18 weeks after surgery.
.jpg) |
| Patrick Ott, MD, PhD and Cathy Wu, MD photo credit– Dana Farber |
The small study involved eight patients who had undergone surgery for advanced melanoma but were considered at high risk of recurrence. Each received injections of NeoVax, a median of 18 weeks after surgery.
Friday, June 26, 2020
Vitamin D may be key to reducing side effect of new cancer drugs
CANCER DIGEST – June 26, 2020 – New research indicates that taking vitamin D supplements may help prevent one of the potentially serious side effects of a revolutionary form of anti-cancer therapy, called checkpoint inhibitors, a new study shows.
The findings are published early online in CANCER, a peer-reviewed journal of the American Cancer Society (ACS).
CANCER DIGEST – June 26, 2020 – New research indicates that taking vitamin D supplements may help prevent one of the potentially serious side effects of a revolutionary form of anti-cancer therapy, called checkpoint inhibitors, a new study shows.
The findings are published early online in CANCER, a peer-reviewed journal of the American Cancer Society (ACS).
Saturday, March 28, 2020
Fewer Americans dying of deadly form of skin cancer
Saturday, May 11, 2019
Sunscreen lotions don't block vitamin D synthesis
 |
| Copyright HYanWong, used under Creative Commons via Wikipedia |
That’s the question researchers at King’s College London asked. Their findings appear in the May 8, 2019 issue of the British Journal of Dermatology.
0 commentsWednesday, May 17, 2017
Nanovaccine shows promise for variety of cancer

Dr. Jinming Gao, Dr. Min Luo, and colleagues developed a
promising nanoparticle vaccine for cancer
CANCER DIGEST – May 17, 2017 – In another approach using nanotechnology to boost the body’s immune system to attack cancer, researchers at the University of Texas Southwestern’s Simmons Cancer Center, have shown in a proof-of-concept study that a nanovaccine extended survival in mouse models of a variety of cancers.
The study published online in the journal Nature Nanotechnology showed effective anti-tumor action in tumor models of melanoma, colorectal cancer, and HPV-related cancers of the cervix, head, neck and anogenital cancers.
 |
| Dr. Jinming Gao, Dr. Min Luo, and colleagues developed a promising nanoparticle vaccine for cancer |
The study published online in the journal Nature Nanotechnology showed effective anti-tumor action in tumor models of melanoma, colorectal cancer, and HPV-related cancers of the cervix, head, neck and anogenital cancers.
Saturday, April 15, 2017
Immunotherapy combination shows promise in advanced melanoma
CANCER DIGEST – April 15, 2017 – A new treatment regiment using an immunotherapy drug combined with an engineered virus injected directly into a melanoma tumor have shown promising results in an early safety trial of the combination.
CANCER DIGEST – April 15, 2017 – A new treatment regiment using an immunotherapy drug combined with an engineered virus injected directly into a melanoma tumor have shown promising results in an early safety trial of the combination.
Friday, January 6, 2017
Promising drug may halt spread of deadly skin cancer
CANCER DIGEST – Jan. 6, 2017 – A potential new drug may block the spread of melanoma, the deadly form of skin cancer, by up to 90 percent, say researchers at the University of Michigan.
The drug developed originally to treat scleroderma, a rare but often fatal autoimmune disease, was found to be effective in blocking the genetic mechanism that triggers melanoma’s spread to other parts of the body.
CANCER DIGEST – Jan. 6, 2017 – A potential new drug may block the spread of melanoma, the deadly form of skin cancer, by up to 90 percent, say researchers at the University of Michigan.
The drug developed originally to treat scleroderma, a rare but often fatal autoimmune disease, was found to be effective in blocking the genetic mechanism that triggers melanoma’s spread to other parts of the body.
Sunday, November 6, 2016
Immunotherapy first may be best for advanced melanoma
CANCER DIGEST – Nov. 6, 2016 – When it comes to melanoma, the deadliest form of skin cancer, what has worked best in recent years have been therapies based on the genetics of the cancer. For melanoma with mutations of the BRAF gene, there have been two approaches. Targeted therapies that seek to selectively kill the cancer and stop it from spreading, and immunotherapies that seek to boost the immune response the cancer.
CANCER DIGEST – Nov. 6, 2016 – When it comes to melanoma, the deadliest form of skin cancer, what has worked best in recent years have been therapies based on the genetics of the cancer. For melanoma with mutations of the BRAF gene, there have been two approaches. Targeted therapies that seek to selectively kill the cancer and stop it from spreading, and immunotherapies that seek to boost the immune response the cancer.
Monday, September 26, 2016
Targeted therapy increases survival in melanoma patients with brain metastases

Copyright: wavebreakmediamicro/123rf
CANCER DIGEST – Sept. 24, 2016 – In a study comparing outcomes for patients whose melanoma skin cancer has spread to their brains, researchers have found that targeted immunological therapies halted the cancer progression and extended survival better than chemotherapy.
In a study published online Sept. 15, in the journal Annals of Oncology, researchers at the Moffitt Cancer Center in Tampa, FL analyzed data from 96 patients with melanoma brain metastases who were treated with radiation therapy within three months of three different types of targeted immune therapies or chemotherapy.
 |
| Copyright: wavebreakmediamicro/123rf |
In a study published online Sept. 15, in the journal Annals of Oncology, researchers at the Moffitt Cancer Center in Tampa, FL analyzed data from 96 patients with melanoma brain metastases who were treated with radiation therapy within three months of three different types of targeted immune therapies or chemotherapy.
Wednesday, July 20, 2016
Skin cancer screenings don’t boost referrals or surgeries
Melanoma can be cured if caught early. Image courtesy
Brown University
CANCER DIGEST – July 20, 2016 – A new study of more than 1,000 primary care melanoma screenings in the western Pennsylvania area suggests that such screenings would not be harmful as some experts had thought.
Melanoma is one of those cancers that can be cured if caught early, which has led to some experts calling for widespread training of primary care providers to conduct screenings at routine visits. Other experts, however, have worried that widespread screening could lead to overtreatment and unnecessary patient distress.
 |
| Melanoma can be cured if caught early. Image courtesy Brown University |
CANCER DIGEST – July 20, 2016 – A new study of more than 1,000 primary care melanoma screenings in the western Pennsylvania area suggests that such screenings would not be harmful as some experts had thought.
Melanoma is one of those cancers that can be cured if caught early, which has led to some experts calling for widespread training of primary care providers to conduct screenings at routine visits. Other experts, however, have worried that widespread screening could lead to overtreatment and unnecessary patient distress.
Thursday, March 10, 2016
Viagra ingredient may fuel skin cancer growth
CANCER DIGEST – March 10, 2016 – The active ingredient in Viagra® can stimulate the growth of existing skin tumors, laboratory and animal studies show. Sildenafil is the ingredient used to treat erectile dysfunction and is the active ingredient in a number of other drugs, which have been on the market since the late 1990s.
Researchers have long debated a possible link between sildenafil and cancer. A long-term study of some 15,000 men in the United States published in 2014 suggested that sildenafil was connected to a higher risk of malignant melanoma.
CANCER DIGEST – March 10, 2016 – The active ingredient in Viagra® can stimulate the growth of existing skin tumors, laboratory and animal studies show. Sildenafil is the ingredient used to treat erectile dysfunction and is the active ingredient in a number of other drugs, which have been on the market since the late 1990s.
Researchers have long debated a possible link between sildenafil and cancer. A long-term study of some 15,000 men in the United States published in 2014 suggested that sildenafil was connected to a higher risk of malignant melanoma.
Wednesday, March 2, 2016
Moles and melanoma what is the link?
CANCER DIGEST – Mar. 2, 2016 – Most patients with melanoma have few moles a new study in the Journal of the American Medical Association shows.
In a Harvard School of Public Health study of 566 patients with melanoma Alan Geller and coauthors looked at the association between age, total moles and abnormal moles to see if there was a correlation between
CANCER DIGEST – Mar. 2, 2016 – Most patients with melanoma have few moles a new study in the Journal of the American Medical Association shows.
In a Harvard School of Public Health study of 566 patients with melanoma Alan Geller and coauthors looked at the association between age, total moles and abnormal moles to see if there was a correlation between
Tuesday, November 3, 2015
First viral therapy for melanoma approved
CANCER DIGEST – Nov. 3, 2015 – The U.S. Food and Drug Administration has approved Imlygic (talimogene laherparepvec), the first FDA-approved anti-cancer virus therapy, for the treatment of melanoma in the skin and lymph nodes.
Imlygic is a genetically modified live herpes virus engineered to kill cancer cells. It is used to treat melanoma tumors that cannot be removed completely by surgery.
CANCER DIGEST – Nov. 3, 2015 – The U.S. Food and Drug Administration has approved Imlygic (talimogene laherparepvec), the first FDA-approved anti-cancer virus therapy, for the treatment of melanoma in the skin and lymph nodes.
Imlygic is a genetically modified live herpes virus engineered to kill cancer cells. It is used to treat melanoma tumors that cannot be removed completely by surgery.
Imlygic is a genetically modified live herpes virus engineered to kill cancer cells. It is used to treat melanoma tumors that cannot be removed completely by surgery.
Friday, June 5, 2015
Melanoma rates double over past 30 years
Thursday, May 14, 2015
A type of vitamin B3 cuts risk of recurring non-melanoma skin cancers
Tuesday, January 20, 2015
Coffee may protect against malignant melanoma
| "A small cup of coffee" by Julius Schorzman - Own work. Licensed under CC BY-SA 2.0 via Wikimedia Commons |
CANCER DIGEST – Jan. 20, 2015 – Could coffee protect against the most lethal type of skin cancer? A new population study shows that it may.
In a large study of 447,347 non-Hispanic white people who filled out dietary questionnaires in 1995 and 1996, researchers led by Erikka Loftfield, MPH of the National Cancer Institute, found that those with the highest intake of caffeinated coffee had a 20 percent lower risk of developing malignant melanoma, the most deadly form of skin cancer. The results appear in today’s JNCI: Journal of the National Cancer Institute.
Monday, December 22, 2014
FDA approves Opdivo for advanced melanoma
CANCER DIGEST – Dec. 22, 2014 – The FDA today granted accelerated approval to Opdivo (nivolumab), a new treatment for patients with melanoma that cannot be treated with surgery or have advanced disease who no longer respond to other drugs.
The FDA granted Opvido breakthrough therapy designation, priority review and orphan product designation because the sponsor demonstrated through preliminary clinical evidence that the drug may offer a substantial improvement over available therapies.
CANCER DIGEST – Dec. 22, 2014 – The FDA today granted accelerated approval to Opdivo (nivolumab), a new treatment for patients with melanoma that cannot be treated with surgery or have advanced disease who no longer respond to other drugs.
The FDA granted Opvido breakthrough therapy designation, priority review and orphan product designation because the sponsor demonstrated through preliminary clinical evidence that the drug may offer a substantial improvement over available therapies.
Tuesday, November 4, 2014
Combination therapy boosts melanoma survival by 50 percent
CANCER DIGEST – Nov. 4, 2014 – Patients with metastatic melanoma who were treated with an unusual combination of an immunotherapy with an immune stimulant survived 50 percent longer compared to patients who received only the immunotherapy.
The study by Dana-Farber Cancer Institute scientists enrolled 245 patients with stage 3 or stage 4 metastatic melanoma who had been treated with other drugs.
CANCER DIGEST – Nov. 4, 2014 – Patients with metastatic melanoma who were treated with an unusual combination of an immunotherapy with an immune stimulant survived 50 percent longer compared to patients who received only the immunotherapy.
The study by Dana-Farber Cancer Institute scientists enrolled 245 patients with stage 3 or stage 4 metastatic melanoma who had been treated with other drugs.
Monday, September 29, 2014
Combination treatment boosts melanoma treatment

OncoLetter YouTube – Sep 29, 2012
CANCER DIGEST – Sept. 25, 2014 – Patients with advanced stage melanoma had their cancer growth halted for an average of almost 10 months when treated with a combination therapy compared to a little more than 6 months for those treated with a single chemotherapy drug.
 |
| OncoLetter YouTube – Sep 29, 2012 |
Thursday, September 4, 2014
FDA grants accelerated approval to Keytruda for melanoma
CANCER DIGEST – Sept. 4, 2014 – The FDA granted fast-track approval to the first of a new class of drugs that promise to hold the key to turning off cancer cells’ ability to evade the body’s immune system.
The drug, pembrolizumab, was approved for treatment of advanced melanoma that is no longer responding to other drugs. It works by blocking a protein called PD-1, which cancer cells produce to restrict the immune system’s ability to attack melanoma cells.
Marketed under the brand name Keytruda by Merck, it is intended for use following treatment with ipillmumab, an immunotherapy drug that acts on a gene mutation, BRAF V600, which triggers signals inside cells to rev up growth.
The FDA granted Keytruda breakthrough-therapy-status because Merck has demonstrated through preliminary clinical evidence that the drug may offer a substantial improvement over available therapies. It allows patients access to the drug while the company conducts clinical trials to confirm the early results.
Keytruda showed its potential effectiveness in 173 clinical trial participants with advanced melanoma whose disease progressed after other treatments failed to halt it. All participants treated with either of two doses of Keytruda responded. In the half of the participants who received Keytruda at the recommended dose of 2 mg/kg, approximately 24 percent had their tumors shrink. This effect lasted at least one to almost nine months and continued beyond nine months in most patients. A similar percentage of patients had their tumors shrink at the 10 mg/kg dose.
The most common side effects of Keytruda were fatigue, cough, nausea, itchy skin (pruritus), rash, decreased appetite, constipation, joint pain (arthralgia) and diarrhea, and has the potential to damage the lungs, colons and hormone-producing glands such as the liver, but this did not occur often.











No comments:
Post a Comment