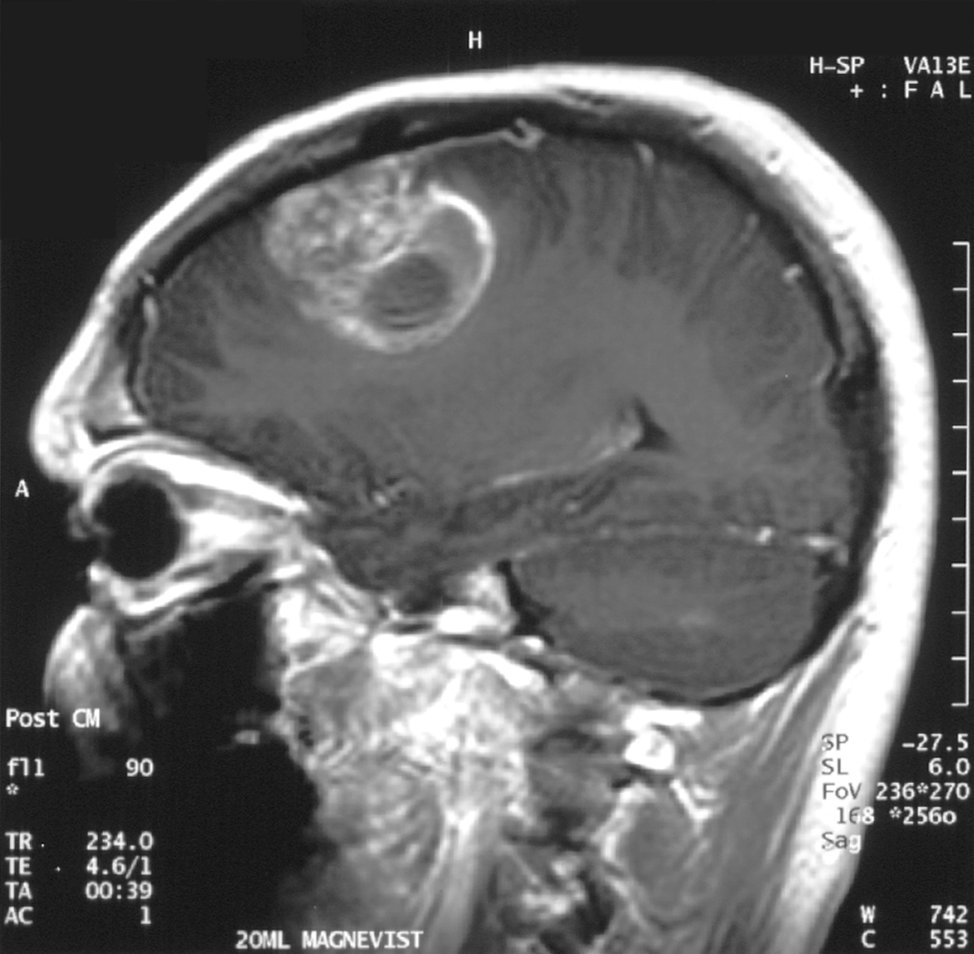
In a study also reported this week in the journal Science Translational Medicine researchers at the Perelman School of Medicine at the University of Pennsylvania used T cells genetically modified to kill cancerous glial cells called glioblastoma.
The T cells are called chimeric antigen receptor or CAR T-cells, which are modified to recognize a specific protein on tumor cells. The FDA approved one such CAR T-cell therapy last week for treatment of a form acute lymphoblastic leukemia (ALL).
In the study, 10 patients with glioblastoma were treated with a CAR T-cell infusion in an early clinical trial aimed at testing safety and effectiveness. The study showed that the cells successfully crossed the blood-brain barrier, a highly selective membrane that often prevents drugs from passing from the blood into the brain, and the experimental cells successfully infiltrated the tumor cells.
The researchers also found, however, that the CAR T-cells met with resistance mechanisms, including an increase in immunosuppression, which may work against the patient and for the tumor.
While no clinical benefit could be definitively shown from the study, one patient did achieve stable disease after 18 months of follow up and remains stable today. Two other patients survive but have disease progression. The other seven patients lived longer than predicted based on the number of previous treatments they received.
“This is an early stage trial, but we are encouraged by the fact that the cells got into the brain, proliferated, and reduced the level of antigen with very little toxicity to the patients,” lead author Donald O’Rourke, MD, a associate professor of neurosurgery at Penn said in a press release. “We can build on this as a therapeutic option for these patients. It gives us clues on what to do next.”
“This is an early stage trial, but we are encouraged by the fact that the cells got into the brain, proliferated, and reduced the level of antigen with very little toxicity to the patients,” lead author Donald O’Rourke, MD, a associate professor of neurosurgery at Penn said in a press release. “We can build on this as a therapeutic option for these patients. It gives us clues on what to do next.”


No comments:
Post a Comment