Saturday, July 16, 2022
Adding an immunotherapy drug may boost survival in Hodgkin Lymphoma patients
Saturday, May 14, 2022
New drug approved for treatment of lymphoma subtype

Image credit – National Cancer Institute
CANCER DIGEST – May 14, 2022 – The FDA has granted provisional approval for a new drug to treat a form of slow-growing cancer called marginal zone lymphoma. The approval is based on early results of a small clinical trial and a secondary study.
The drug, zanubrutinib, belongs to a new class of drug that blocks a certain enzyme that plays a crucial role in allowing the cancer to survive and grow. The results of the early clinical trial led by Tycel Phillips, MD, of the University of Michigan appear in the April 7, 2022 journal Blood Advances.
 |
| Image credit – National Cancer Institute |
The drug, zanubrutinib, belongs to a new class of drug that blocks a certain enzyme that plays a crucial role in allowing the cancer to survive and grow. The results of the early clinical trial led by Tycel Phillips, MD, of the University of Michigan appear in the April 7, 2022 journal Blood Advances.
Tuesday, May 3, 2022
Researchers boost effectiveness of CAR T therapy for AML
Sunday, December 26, 2021
New treatment for GVHD could benefit some COVID-19 patients
Sunday, November 21, 2021
Surviving childhood cancer carries long-term health effects
Saturday, November 6, 2021
Younger patient with lymphoma subtype live longer with ibrutinib added to therapy
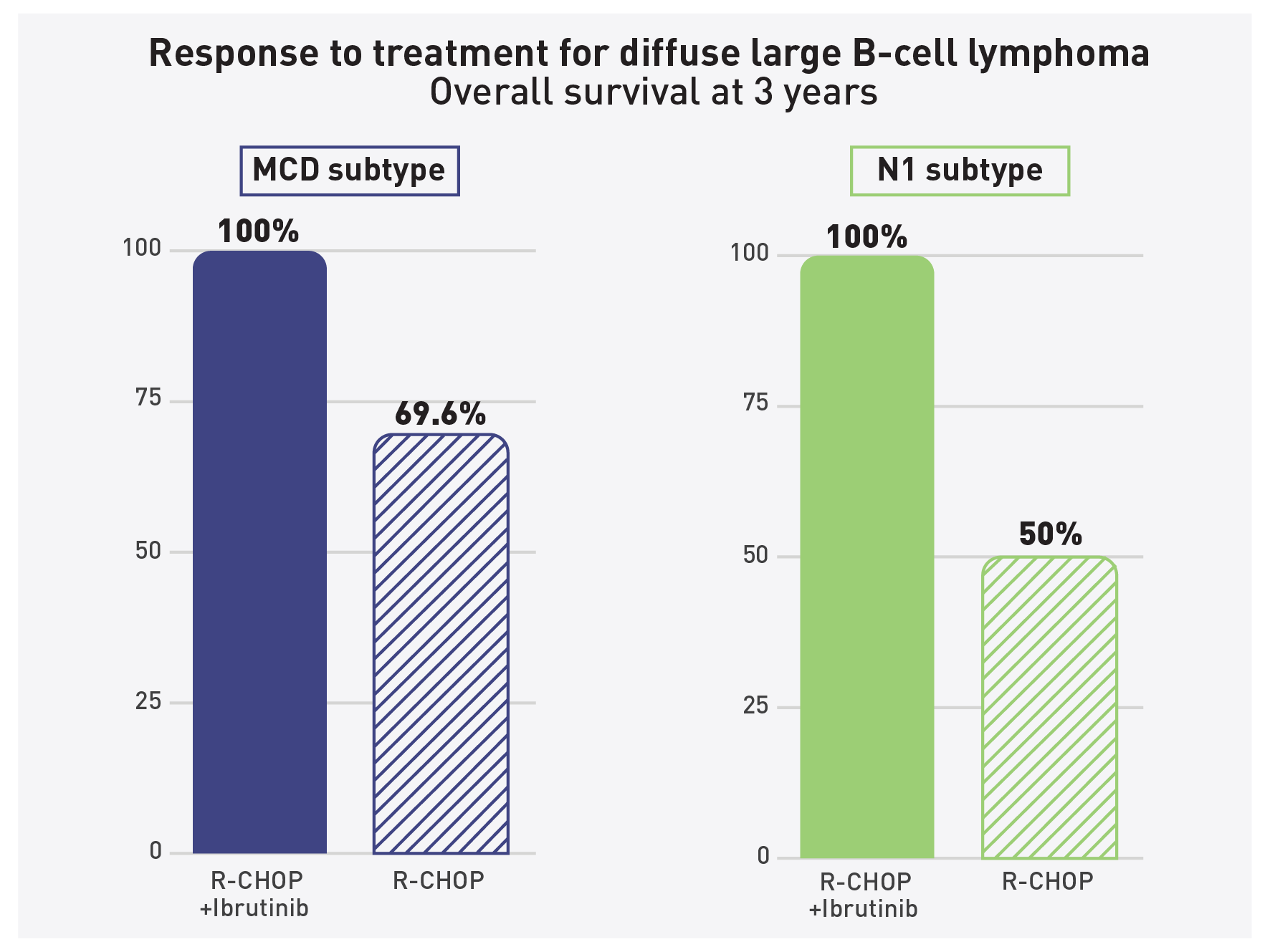
The addition of ibrutinib to R-CHOP chemo-
therapy improved overall survival among
trial participants aged 60 and younger. –
Credit NCI
CANCER DIGEST – Nov. 6, 2021 – In a surprising result, researchers have found that adding ibrutinib (Imbruvica®), a targeted therapy, to chemotherapy can improve survival for young people with a type of the most common form of lymphoma, a new analysis in the Nov. 4, 2021 Cancer Cell shows.
The results stem from the PHOENIX clinical trial testing the combination of standard chemotherapy with the targeted therapy ibrutinib in patients with diffuse large B-cell lymphoma (DLBCL), the most common type of lymphoma comprising 40 percent of all lymphomas in the world.
 |
| The addition of ibrutinib to R-CHOP chemo- therapy improved overall survival among trial participants aged 60 and younger. – Credit NCI |
The results stem from the PHOENIX clinical trial testing the combination of standard chemotherapy with the targeted therapy ibrutinib in patients with diffuse large B-cell lymphoma (DLBCL), the most common type of lymphoma comprising 40 percent of all lymphomas in the world.
Monday, May 3, 2021
T cell therapy effective against painful complication of stem cell transplants
CANCER DIGEST – May 3, 2021 – An infusion of specific T cells from healthy donors were safe and effective as an off-the-shelf therapy for a common painful complication of allogeneic (donor) stem cell patients, a new clinical trial shows. The study appears in the April 30, 2021 Journal of Clinical Oncology.
CANCER DIGEST – May 3, 2021 – An infusion of specific T cells from healthy donors were safe and effective as an off-the-shelf therapy for a common painful complication of allogeneic (donor) stem cell patients, a new clinical trial shows. The study appears in the April 30, 2021 Journal of Clinical Oncology.
Saturday, April 10, 2021
Dual targeted CAR T-cell approach shows promise of reducing resistant relapse

The diagram above represents the process of chimeric antigen receptor T-cell therapy (CAR).
Image credit – Reyasingh56 via Wikipedia Creative Commons license.
CANCER DIGEST – April 10, 2021 – Early results from a small clinical trial involving 5 patients to test safety of a dual targeted immunotherapy approach has shown promise of minimizing treatment resistance, a group of UCLA Jonsson Comprehensive Cancer Center researchers report.
 |
| The diagram above represents the process of chimeric antigen receptor T-cell therapy (CAR). Image credit – Reyasingh56 via Wikipedia Creative Commons license. |
Saturday, March 20, 2021
Three-fold increase in remission for multiple myeloma patients receiving new version CAR T-cell therapy

Illustration of CAR T-cell therapy courtesy of UT Southwestern
Medical Center
CANCER DIGEST – March 20, 2021 – A new version of CAR T-cell therapy provided a three-fold increase in length of remission for patients with multiple myeloma who have relapsed multiple times, according to results of an international study.
The study conducted in the US, Canada and Europe involved 128 patients with multiple myeloma, a cancer of the bone marrow that steadily reduces the ability of the immune system cells to protect body from infections. The results were published in the Feb. 25, 2021 New England Journal of Medicine.
 |
| Illustration of CAR T-cell therapy courtesy of UT Southwestern Medical Center |
The study conducted in the US, Canada and Europe involved 128 patients with multiple myeloma, a cancer of the bone marrow that steadily reduces the ability of the immune system cells to protect body from infections. The results were published in the Feb. 25, 2021 New England Journal of Medicine.
Friday, December 4, 2020
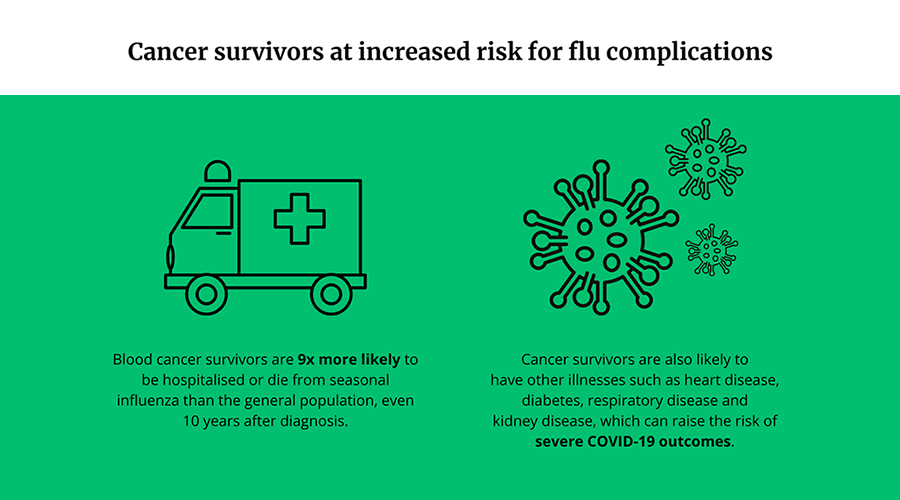
Cancer survivors more likely to be hospitalized or die of seasonal flu
Saturday, September 26, 2020
Immune depleting cancer therapy poses highest risk of dying from COVID-19

Trisha Wise-Draper, MD, PhD, in her lab in
the Vontz Center for Molecular Studies.
Photo credit/Colleen Kelley/UC Creative + Brand
CANCER DIGEST – Sept. 26, 2020 – Researchers have known from the earliest phase of the COVID-19 pandemic that cancer patients were at greater risk of dying from the virus. A new study shows that cancer patients who have recently undergone a certain type of cancer treatment within one to three months prior to infection were at the highest risk of dying of the virus.
 |
| Trisha Wise-Draper, MD, PhD, in her lab in the Vontz Center for Molecular Studies. Photo credit/Colleen Kelley/UC Creative + Brand |
Friday, July 17, 2020
Researchers say their cancer vaccine is ready for human trials

The Vaccine Research Team at Translational Research Institute, Australia says
they are ready to begin human clinical trials of their cancer vaccine.
Image courtesy TRI
CANCER DIGEST – July 17, 2020 – Scientists are ready to begin clinical trials for a new cancer vaccine in humans they say has the potential to treat a variety of blood cancers, following results of preclinical trials appearing in the journal Clinical and Translational Immunology.
 |
| The Vaccine Research Team at Translational Research Institute, Australia says they are ready to begin human clinical trials of their cancer vaccine. Image courtesy TRI |
CANCER DIGEST – July 17, 2020 – Scientists are ready to begin clinical trials for a new cancer vaccine in humans they say has the potential to treat a variety of blood cancers, following results of preclinical trials appearing in the journal Clinical and Translational Immunology.
Saturday, April 4, 2020
Cancer drug a potential anti-COVID-19 treatment for people with blood cancers
Saturday, October 12, 2019
Viagra may speed stem cell harvesting for bone marrow transplants

Diagram shows standard regimen (left) compared to new
Viagra regimen (right) – Credit Smith-Brendan, et al,
Stem Cell Reports 2019
CANCER DIGEST – Oct. 12, 2019 – A combination of Viagra and a second drug called Plerixafor speeds production and mobilization of blood-forming stem cells needed for bone marrow transplants, new research shows.
The new approach to harvesting stem cells involves a single oral dose of Viagra followed two hours later by a single injection of Plerixafor. The study published Oct. 10, 2019 in Stem Cell Reports showed that the method mobilized enough stem cells from the bone marrow in just 2 hours.
 |
| Diagram shows standard regimen (left) compared to new Viagra regimen (right) – Credit Smith-Brendan, et al, Stem Cell Reports 2019 |
The new approach to harvesting stem cells involves a single oral dose of Viagra followed two hours later by a single injection of Plerixafor. The study published Oct. 10, 2019 in Stem Cell Reports showed that the method mobilized enough stem cells from the bone marrow in just 2 hours.
Saturday, August 24, 2019
New combination therapy effective for multiple myeloma
CANCER DIGEST – Aug. 24, 2019 – A new combination oral therapy has been shown to halt or reverse a type of cancer of the bone marrow, according to results of a small clinical trial.
Multiple myeloma is a cancer of the marrow cells that make plasma and help fight infections. When these cells become cancerous they grow out of control and crowd out functioning immune system cells. The disease can damage the bones, immune system, kidneys, and red blood cell count.
CANCER DIGEST – Aug. 24, 2019 – A new combination oral therapy has been shown to halt or reverse a type of cancer of the bone marrow, according to results of a small clinical trial.
Multiple myeloma is a cancer of the marrow cells that make plasma and help fight infections. When these cells become cancerous they grow out of control and crowd out functioning immune system cells. The disease can damage the bones, immune system, kidneys, and red blood cell count.
Saturday, January 19, 2019
Antibodies may better control viral complication of marrow transplants
0 commentsSaturday, January 12, 2019
Study shows new leukemia drug is better than standard therapy
Malignant white blood cells
crowd out normal cells in CLL
– image courtesy American
Society of Hematology
In a study of 547 patients with the most common form of leukemia, researchers have found that newly approved drug is both more effective and easier to take than conventional therapy.
The study led by Scott Smith, MD, PhD of Loyola Medicine and Loyola University Chicago Stritch School of Medicine, the researchers enrolled 547 patients with chronic lymphocytic leukemia (CLL) at 219 cancer centers in the U.S. and Canada. All participants were over the age of 65 and randomly assigned one of three treatments. The standard treatment of bendamustine plus rituximab, ibrutinib (Imbruvica®) alone, or ibrutinib plus rituximab.
| Malignant white blood cells crowd out normal cells in CLL – image courtesy American Society of Hematology |
The study led by Scott Smith, MD, PhD of Loyola Medicine and Loyola University Chicago Stritch School of Medicine, the researchers enrolled 547 patients with chronic lymphocytic leukemia (CLL) at 219 cancer centers in the U.S. and Canada. All participants were over the age of 65 and randomly assigned one of three treatments. The standard treatment of bendamustine plus rituximab, ibrutinib (Imbruvica®) alone, or ibrutinib plus rituximab.
Wednesday, December 26, 2018
Maintenance therapy prolongs remission, survival times for myeloma
CANCER DIGEST – Dec. 26, 2018 – A new study shows that patients with newly diagnosed myeloma have extended remissions and better survival when given ongoing therapy with the drug lenalidomide (Revlimid®) according to researchers.
Myeloma is a bone marrow cancer that results in over production of plasma cells resulting in a weakened immune system and interferes with normal production and function of red and white blood cells.
CANCER DIGEST – Dec. 26, 2018 – A new study shows that patients with newly diagnosed myeloma have extended remissions and better survival when given ongoing therapy with the drug lenalidomide (Revlimid®) according to researchers.
Myeloma is a bone marrow cancer that results in over production of plasma cells resulting in a weakened immune system and interferes with normal production and function of red and white blood cells.
Saturday, November 10, 2018
New combination treatment shows promise for AML
Saturday, August 11, 2018
CLL patients need to be monitored for developing melanoma
CANCER DIGEST – Aug. 11, 2018 – People with a form of leukemia called chronic lymphocytic leukemia (CLL) have a substantially higher risk of melanoma, the deadly form of skin cancer, researcher report.
Although the higher risk of melanoma for CLL patients had been known, a full analysis of the relationship has never been done before researchers led by Clive Zent, MD of the Wilmot cancer Institute reported findings in the August 2018 journal Leukemia Research.
CANCER DIGEST – Aug. 11, 2018 – People with a form of leukemia called chronic lymphocytic leukemia (CLL) have a substantially higher risk of melanoma, the deadly form of skin cancer, researcher report.
Although the higher risk of melanoma for CLL patients had been known, a full analysis of the relationship has never been done before researchers led by Clive Zent, MD of the Wilmot cancer Institute reported findings in the August 2018 journal Leukemia Research.
Although the higher risk of melanoma for CLL patients had been known, a full analysis of the relationship has never been done before researchers led by Clive Zent, MD of the Wilmot cancer Institute reported findings in the August 2018 journal Leukemia Research.
Thursday, August 31, 2017
FDA approves first 'living drug' therapy for cancer
CANCER DIGEST – Aug. 31, 2017 – The US Food and Drug Administration approved the first treatment for cancer based on genetic engineering of the patient’s own immune system.
While it has been referred to as gene therapy, the new treatment does not actually change or replace the genes that cause the cancer, rather it alters genes of the immune system to better recognize and eliminate the cancer. In that sense it is the first FDA-approved gene therapy in that it reprograms the patient’s own cells to attack the cancer.
CANCER DIGEST – Aug. 31, 2017 – The US Food and Drug Administration approved the first treatment for cancer based on genetic engineering of the patient’s own immune system.
While it has been referred to as gene therapy, the new treatment does not actually change or replace the genes that cause the cancer, rather it alters genes of the immune system to better recognize and eliminate the cancer. In that sense it is the first FDA-approved gene therapy in that it reprograms the patient’s own cells to attack the cancer.
While it has been referred to as gene therapy, the new treatment does not actually change or replace the genes that cause the cancer, rather it alters genes of the immune system to better recognize and eliminate the cancer. In that sense it is the first FDA-approved gene therapy in that it reprograms the patient’s own cells to attack the cancer.
Saturday, August 12, 2017
FDA approves two drugs for certain types of AML

A scanning electron
microscope image
from normal circulating
human blood. – Wikipedia
CANCER DIGEST – Aug. 12, 2017 – The FDA approved two new drugs for treatment of specific types of acute myeloid leukemia (AML), a rapidly progressing cancer that forms in the bone marrow.
Vyxeos was approved for two types of AML, newly diagnosed therapy-related AML called t-AML, and myelodysplasia-related changes changes called ALM-MRC.
 |
A scanning electron
microscope image
from normal circulating
human blood. – Wikipedia
|
Vyxeos was approved for two types of AML, newly diagnosed therapy-related AML called t-AML, and myelodysplasia-related changes changes called ALM-MRC.
Saturday, July 15, 2017
FDA panel recommends approval for gene-altering therapy
Saturday, June 24, 2017
Promising new drug for relapsed AML

Bone marrow aspirate showing
acute myeloid leukemia, arrows
indicate Auer rods - Wikipedia
CANCER DIGEST – June 24, 2017 – Researchers have found a drug that inhibits a particular mutation in a deadly form of acute myeloid leukemia (AML) with promise of producing longer survival.
The sub-type of AML has a mutation in a gene called FLT3, found in about 30 percent of patients’ leukemia cells.
 |
| Bone marrow aspirate showing acute myeloid leukemia, arrows indicate Auer rods - Wikipedia |
The sub-type of AML has a mutation in a gene called FLT3, found in about 30 percent of patients’ leukemia cells.
Wednesday, May 10, 2017
Reprogramming T cells on the fly to fight cancer

Dr. Matthias Stephan
CANCER DIGEST – May 10, 2017 – The idea of using the body’s own immune system to halt cancer has a long history of research approaches. One of the most promising in that vein over the last decade has been immunotherapy, and most recently the use of T cells engineered with chimeric antigen receptors or CAR T cells.
| Dr. Matthias Stephan |
Monday, December 26, 2016
Herpes virus linked to most common childhood cancer
CANCER DIGEST – Dec. 26, 2016 – Newborns with a common virus in the herpes family may have an increased risk of developing acute lymphocytic leukemia (ALL), according to new research. The study suggests the risk is even greater in Hispanic children.
The new research led by Stephen Francis, PhD, assistant professor of epidemiology at the University of Nevada and University of California, San Francisco was published online in Blood, the Journal of the American Society of Hematology (ASH).
CANCER DIGEST – Dec. 26, 2016 – Newborns with a common virus in the herpes family may have an increased risk of developing acute lymphocytic leukemia (ALL), according to new research. The study suggests the risk is even greater in Hispanic children.
The new research led by Stephen Francis, PhD, assistant professor of epidemiology at the University of Nevada and University of California, San Francisco was published online in Blood, the Journal of the American Society of Hematology (ASH).
Sunday, November 13, 2016
People with genetic disorder linked to long life have increased death rate from a common cancer drug
CANCER DIGEST – Nov. 13, 2016 – Cancer patients with a genetic disorder that has been linked to long life, ironically may be twice as likely to die when treated with a common chemotherapy drug, a new analysis shows.
Led by George McDonald, MD a gastroenterology researcher at Fred Hutchinson Cancer Research Center in Seattle, researchers analyzed the records of 3500 marrow transplant patients over a ten-year period between 1991 and 2011. The records analyzed included people who had been treated with busulfan as part of a chemotherapy regimen prior to bone marrow transplantation for blood cancers like leukemia or lymphoma.
CANCER DIGEST – Nov. 13, 2016 – Cancer patients with a genetic disorder that has been linked to long life, ironically may be twice as likely to die when treated with a common chemotherapy drug, a new analysis shows.
Led by George McDonald, MD a gastroenterology researcher at Fred Hutchinson Cancer Research Center in Seattle, researchers analyzed the records of 3500 marrow transplant patients over a ten-year period between 1991 and 2011. The records analyzed included people who had been treated with busulfan as part of a chemotherapy regimen prior to bone marrow transplantation for blood cancers like leukemia or lymphoma.
Saturday, September 17, 2016
Researchers hopeful for improved T cell therapy for non-Hodgkin lymphoma

Dr. Stan Riddell, is a senior researcher for the trial –
Fred Hutch file photo
CANCER DIGEST – Sept. 17, 2016 – In an early clinical trial designed to determine the optimal safe dose, researchers at the Fred Hutchinson Cancer Research Center in Seattle, WA have seen promising results for an anti-cancer immune cell engineered from the patient’s own immune system.
The early results of the engineered T cell, called JCAR014 were from what is called a dose-finding trial in patients with non-Hodgkin lymphoma (NHL) published in the journal Science Translational Medicine.
| Dr. Stan Riddell, is a senior researcher for the trial – Fred Hutch file photo |
CANCER DIGEST – Sept. 17, 2016 – In an early clinical trial designed to determine the optimal safe dose, researchers at the Fred Hutchinson Cancer Research Center in Seattle, WA have seen promising results for an anti-cancer immune cell engineered from the patient’s own immune system.
The early results of the engineered T cell, called JCAR014 were from what is called a dose-finding trial in patients with non-Hodgkin lymphoma (NHL) published in the journal Science Translational Medicine.
Saturday, August 13, 2016
Venetoclax safe, shows promise for AML
 CANCER DIGEST – Aug. 12, 2016 – In a small trial with 32 patients with acute myelogenous leukemia (AML) that no longer responded to chemotherapy, four patients treated with a new drug achieved a complete response, meaning no sign of cancer, and six had no cancer cells present, but continued to have signs of the cancer in their blood.
CANCER DIGEST – Aug. 12, 2016 – In a small trial with 32 patients with acute myelogenous leukemia (AML) that no longer responded to chemotherapy, four patients treated with a new drug achieved a complete response, meaning no sign of cancer, and six had no cancer cells present, but continued to have signs of the cancer in their blood.
That is enough of a response to the drug Venclexta (venetoclax) to give researchers hope of adding a new approach to treating acute myelogenous leukemia, a particularly deadly type of blood cancer in which only about 27 percent survive five years after diagnosis.
 CANCER DIGEST – Aug. 12, 2016 – In a small trial with 32 patients with acute myelogenous leukemia (AML) that no longer responded to chemotherapy, four patients treated with a new drug achieved a complete response, meaning no sign of cancer, and six had no cancer cells present, but continued to have signs of the cancer in their blood.
CANCER DIGEST – Aug. 12, 2016 – In a small trial with 32 patients with acute myelogenous leukemia (AML) that no longer responded to chemotherapy, four patients treated with a new drug achieved a complete response, meaning no sign of cancer, and six had no cancer cells present, but continued to have signs of the cancer in their blood.
That is enough of a response to the drug Venclexta (venetoclax) to give researchers hope of adding a new approach to treating acute myelogenous leukemia, a particularly deadly type of blood cancer in which only about 27 percent survive five years after diagnosis.
Monday, April 11, 2016
New drug approved for chronic lymphocytic leukemia
CANCER DIGEST – April 11, 2016 – The FDA today approved a new drug for treatment of patients with a form of chronic lymphocytic leukemia (CLL).
The drug manufactured by AbbVie Inc. of North Chicago, Illinois, is called Venclexta (venetoclax) and is for the treatment of patients with CLL who have a genetic abnormality called 17p deletion and who have been treated with a least one prior therapy.
CANCER DIGEST – April 11, 2016 – The FDA today approved a new drug for treatment of patients with a form of chronic lymphocytic leukemia (CLL).
The drug manufactured by AbbVie Inc. of North Chicago, Illinois, is called Venclexta (venetoclax) and is for the treatment of patients with CLL who have a genetic abnormality called 17p deletion and who have been treated with a least one prior therapy.
Saturday, March 19, 2016
New drug shows promise for drug resistant leukemia

A scanning electron
microscope image
from normal circulating
human blood. – Wikipedia
CANCER DIGEST – March 19, 2016 – Researchers have developed a compound that shows promise for extending survival in patients with a drug-resistant form of leukemia.
Adult acute myeloid leukemia (AML) is a cancer of theblood and bone marrow that usually gets worse quickly if it is not treated. It has a poor prognosis, with survival rates between 60 percent and 70 percent in children and less than 50 percent in adults.
 |
A scanning electron
microscope image
from normal circulating
human blood. – Wikipedia
|
CANCER DIGEST – March 19, 2016 – Researchers have developed a compound that shows promise for extending survival in patients with a drug-resistant form of leukemia.
Adult acute myeloid leukemia (AML) is a cancer of theblood and bone marrow that usually gets worse quickly if it is not treated. It has a poor prognosis, with survival rates between 60 percent and 70 percent in children and less than 50 percent in adults.
Tuesday, February 16, 2016
Genetically modified immunotherapy shows ‘unprecedented’ success

Scanning electron micrograph
of a human T cell Credit: NIAID
CANCER DIGEST – Feb. 16, 2016 – Immune system cells engineered to attack cancer cells raised optimistic “alerts” in the mainstream media this week, as a researcher reported 94 percent of patients with advanced acute lymphoblastic leukemia (ALL) saw their symptoms disappear.
The results were reported at the just concluded American Association for the Advancement of Science conference in Washington, DC, by Dr. Stan Ridell, of Fred Hutchinson Cancer Research Center in Seattle, WA.
 |
| Scanning electron micrograph of a human T cell Credit: NIAID |
CANCER DIGEST – Feb. 16, 2016 – Immune system cells engineered to attack cancer cells raised optimistic “alerts” in the mainstream media this week, as a researcher reported 94 percent of patients with advanced acute lymphoblastic leukemia (ALL) saw their symptoms disappear.
The results were reported at the just concluded American Association for the Advancement of Science conference in Washington, DC, by Dr. Stan Ridell, of Fred Hutchinson Cancer Research Center in Seattle, WA.
Saturday, November 7, 2015
Girl undergoes first-ever ‘gene-editing’ treatment

Layla, shown here at 16 months,
is the first patient to receive new
treatment
CANCER DIGEST – Nov. 7, 2015 – A one-year-old girl in London, England with leukemia is now cancer free and doing well as a result of a new treatment that uses ‘molecular scissors’ to edit genes and create designer immune cells.
The girl, named Layla, had relapsed acute lymphoblastic leukemia (ALL) that had failed to respond to conventional therapies and had limited treatment options available.
The patient’s parents were keen to try the treatment. Mother, Lisa, says: "We didn’t want to accept palliative care and so we asked the doctors to try anything for our daughter, even if it hadn’t been tried before."
 |
| Layla, shown here at 16 months, is the first patient to receive new treatment |
CANCER DIGEST – Nov. 7, 2015 – A one-year-old girl in London, England with leukemia is now cancer free and doing well as a result of a new treatment that uses ‘molecular scissors’ to edit genes and create designer immune cells.
The girl, named Layla, had relapsed acute lymphoblastic leukemia (ALL) that had failed to respond to conventional therapies and had limited treatment options available.
The patient’s parents were keen to try the treatment. Mother, Lisa, says: "We didn’t want to accept palliative care and so we asked the doctors to try anything for our daughter, even if it hadn’t been tried before."
Wednesday, October 7, 2015
Imperfect match may be just as good as perfect match for blood cancers

A scanning electron
microscope image
from normal circulating
human blood. – Wikipedia
CANCER DIGEST – Oct. 7, 2015 – Using a half-matched donor bone marrow transplant may be just as good as a full match for treating blood cancers like leukemia and lymphomas, new research shows.
In the first study to compare the gold standard full-match to a half-match transplant using an identical protocol, researchers at the Thomas Jefferson Kimmel Cancer Center have shown three years after transplant approximately 70 percent of the patients in both groups were still alive and cancer free.
 |
A scanning electron
microscope image
from normal circulating
human blood. – Wikipedia
|
CANCER DIGEST – Oct. 7, 2015 – Using a half-matched donor bone marrow transplant may be just as good as a full match for treating blood cancers like leukemia and lymphomas, new research shows.
In the first study to compare the gold standard full-match to a half-match transplant using an identical protocol, researchers at the Thomas Jefferson Kimmel Cancer Center have shown three years after transplant approximately 70 percent of the patients in both groups were still alive and cancer free.
Sunday, September 27, 2015
New hope for older Hodgkin lymphoma patients

They lymph node system
throughout the body is part
of the immune system.
CANCER DIGEST – Sept. 27, 2015 – A new engineered antibody drug may offer hope for people over 60 with Hodgkin lymphoma, early results from a clinical trial show.
In the small trial of 27 people whose average age was 78 with Hodgkin lymphoma, and were not able or willing to undergo standard chemotherapy, 24 achieved an objective response, meaning the drug halted the progression of the cancer or caused it to go into remission.
 |
| They lymph node system throughout the body is part of the immune system. |
CANCER DIGEST – Sept. 27, 2015 – A new engineered antibody drug may offer hope for people over 60 with Hodgkin lymphoma, early results from a clinical trial show.
In the small trial of 27 people whose average age was 78 with Hodgkin lymphoma, and were not able or willing to undergo standard chemotherapy, 24 achieved an objective response, meaning the drug halted the progression of the cancer or caused it to go into remission.
Monday, July 20, 2015
T-cell therapy slows plasma cancer
The micrograph shows abundant
cancerous plasma cells. – by Nephron
via Wikimedia
CANCER DIGEST – July 20, 2015 – Results from a clinical trial testing a new therapy for multiple myeloma demonstrated a clinical response in 80 percent of patients with advanced disease who had undergone a stem cell transplants. A clinical response indicates a therapeutic effect for the treatment that uses a person's own immune system to recognize and destroy cancer cells.
 |
| The micrograph shows abundant cancerous plasma cells. – by Nephron via Wikimedia |
CANCER DIGEST – July 20, 2015 – Results from a clinical trial testing a new therapy for multiple myeloma demonstrated a clinical response in 80 percent of patients with advanced disease who had undergone a stem cell transplants. A clinical response indicates a therapeutic effect for the treatment that uses a person's own immune system to recognize and destroy cancer cells.
Tuesday, June 30, 2015
Experimental combination treatment halts rare leukemia

A prolymphocyte is a white
blood cell – Wikipedia
CANCER DIGEST – June 30, 2015 –An experimental new treatment approach for a rare, deadly leukemia can send the disease into remission even in patients for whom the standard therapy has failed, buying them more time to have the stem cell transplant that could save their lives, a small pilot study has found.
The new approach to battling T-cell prolymphocytic leukemia uses a combination of two drugs already approved for cancer and other therapies, alemtuzumab and cladribine.
 |
| A prolymphocyte is a white blood cell – Wikipedia |
CANCER DIGEST – June 30, 2015 –An experimental new treatment approach for a rare, deadly leukemia can send the disease into remission even in patients for whom the standard therapy has failed, buying them more time to have the stem cell transplant that could save their lives, a small pilot study has found.
The new approach to battling T-cell prolymphocytic leukemia uses a combination of two drugs already approved for cancer and other therapies, alemtuzumab and cladribine.
Thursday, April 9, 2015
Tumor DNA in blood predicts recurrence of lymphoma
CANCER DIGEST – April 9, 2015 – Patients who have had the most common form of lymphoma might learn if their cancer has returned earlier with a blood test, researchers say.
In a study that followed 126 patients who achieved complete remission of their diffuse large B-cell lymphoma (DLBCL) for many years, researchers found that measuring blood levels of the tumor’s DNA enabled detection of microscopic disease before it could be seen on computerized tomography CT scans.
CANCER DIGEST – April 9, 2015 – Patients who have had the most common form of lymphoma might learn if their cancer has returned earlier with a blood test, researchers say.
In a study that followed 126 patients who achieved complete remission of their diffuse large B-cell lymphoma (DLBCL) for many years, researchers found that measuring blood levels of the tumor’s DNA enabled detection of microscopic disease before it could be seen on computerized tomography CT scans.
Thursday, December 4, 2014
Drug for rare form of leukemia given FDA nod

Amgen's blinatumomab allows T-cells to
attach to an immature B-cell and destroy it.
CANCER DIGEST – Dec. 4, 2014 – The FDA has approved Blincyto (blinatumomab) to treat patients with a rare type of leukemia, called Philadelphia chromosome-negative precursor B-cell acute lymphoblastic leukemia (B-cell ALL).
Precursor B-cell ALL is a rapidly growing type of leukemia in which the bone marrow makes too many immature B-cells that are not yet functional in the immune system. The National Cancer Institute estimates that 6,020 Americans will be diagnosed with this form of leukemia and 1,440 will die from it in 2014. The Philadelphia chromosome is an abnormality that sometimes occurs in the bone marrow cells of leukemia patients and is linked to chronic myelogenous leukemia (CML).
 |
| Amgen's blinatumomab allows T-cells to attach to an immature B-cell and destroy it. |
CANCER DIGEST – Dec. 4, 2014 – The FDA has approved Blincyto (blinatumomab) to treat patients with a rare type of leukemia, called Philadelphia chromosome-negative precursor B-cell acute lymphoblastic leukemia (B-cell ALL).
Precursor B-cell ALL is a rapidly growing type of leukemia in which the bone marrow makes too many immature B-cells that are not yet functional in the immune system. The National Cancer Institute estimates that 6,020 Americans will be diagnosed with this form of leukemia and 1,440 will die from it in 2014. The Philadelphia chromosome is an abnormality that sometimes occurs in the bone marrow cells of leukemia patients and is linked to chronic myelogenous leukemia (CML).
Wednesday, August 20, 2014
Leukemia drug may slow metastasis in skin, breast and other cancers
CANCER DIGEST – Aug. 20, 2014 – A drug used to treat leukemia may be useful in slowing or halting cancer spread in other cancers, a new study shows.
The drug, Dasatinib is a type of drug called a protein tyrosine kinase inhibitor (TKI). Tyrosine kinases are proteins that act as chemical messengers to stimulate cancer cells to grow.
CANCER DIGEST – Aug. 20, 2014 – A drug used to treat leukemia may be useful in slowing or halting cancer spread in other cancers, a new study shows.
The drug, Dasatinib is a type of drug called a protein tyrosine kinase inhibitor (TKI). Tyrosine kinases are proteins that act as chemical messengers to stimulate cancer cells to grow.
Tuesday, July 8, 2014
AMGEN drug for ALL receives FDA’s breakthrough designation

AboutKidsHealth.ca video
CANCER DIGEST – July 8, 2014 – The FDA granted breakthrough therapy status to blinatumomab for the most common form of acute lymphoblastic leukemia (ALL). The cancer results from too many immature white blood cells in the blood and bone marrow. To get breakthrough designation, a drug must show preliminary clinical evidence indicating the drug may demonstrate substantial improvement over existing therapies. A total of 82 of 189 (43 percent) patients treated with blinatumomab achieved a complete remission or a partial hematological recovery. The results were presented at the 2014 ASCO Annual Meeting in June. Blinatumomab is an engineered antibody that directs T-cells in the immune system to attack cells that produce certain molecules found on the surface of the abnormal ALL white blood cells. Sean E. Harper, MD, executive vice president, Research and Development at Amgen, which makes the drug, said in a press release that the company is currently recruiting for a large clinical trial that will compare blinatumomab to standard chemotherapy, and plans to enroll 400 patients over the next two to three years.
 |
| AboutKidsHealth.ca video |
CANCER DIGEST – July 8, 2014 – The FDA granted breakthrough therapy status to blinatumomab for the most common form of acute lymphoblastic leukemia (ALL). The cancer results from too many immature white blood cells in the blood and bone marrow. To get breakthrough designation, a drug must show preliminary clinical evidence indicating the drug may demonstrate substantial improvement over existing therapies. A total of 82 of 189 (43 percent) patients treated with blinatumomab achieved a complete remission or a partial hematological recovery. The results were presented at the 2014 ASCO Annual Meeting in June. Blinatumomab is an engineered antibody that directs T-cells in the immune system to attack cells that produce certain molecules found on the surface of the abnormal ALL white blood cells. Sean E. Harper, MD, executive vice president, Research and Development at Amgen, which makes the drug, said in a press release that the company is currently recruiting for a large clinical trial that will compare blinatumomab to standard chemotherapy, and plans to enroll 400 patients over the next two to three years.
Wednesday, May 28, 2014
Promising target for treating leukemia
 |
| Courtesy – Institute for Research in Immunology and Cancer of Université de Montréal |
CANCER DIGEST – May 28, 2014 – A group of researchers at the Institute for Research in Immunology and Cancer of Université de Montréal have identified a gene responsible for tumor progression in leukemia. The gene, known as Brg1, plays a key role in leukemia stem cells that are the root cause of the disease, and lead to treatment resistance and relapse. The researchers led by Julie Lessard say that when the gene is removed from the leukemic cells they no longer divide, thus effectively shutting down the disease progression. If drugs can be developed that block the drug, it might lead to effective treatment for leukemia and other cancers. Their study was published in the journal Blood.
Tuesday, May 27, 2014
Phase 2 results in CPXcitement for AML
 |
| YouTube courtesy Leukemia and Lymphoma Society of Canada |
CANCER DIGEST – May 27, 2014 – Results from a small phase 2 clinical trial of a new chemotherapy for acute myeloid leukemia (AML) were favorable enough that researchers at the Moffitt Cancer Center in Tampa, FL, are now recruiting for a larger comparison clinical trial to determine effectiveness. AML is an aggressive blood cancer with very low rates of treatment success. The new drug called CPX-351 “encapsulates” two chemotherapy drugs, cytarabine and daunorubici, in a lipid shell that maintains the effective ratio of the two drugs until delivery to the tumor. In the early trial of 126 newly diagnosed AML patients those who received CPX-351 and whose AML arose out of a previously diagnosed hematologic disorder, such as myelodysplastic syndrome, had higher rates of remission and survived longer, although the objective of the trial was to determine the safest, most effective dose, not survival. The phase 3 CPX-351 clinical trial is currently open and recruiting patients and is designed to measure survival. Those interested in learning more about study should contact Clinical Research Coordinator Nancy Hillgruber atNancy.Hillgruber@Moffitt.org. The results from the phase 2 study appeared in the May 22, 2014 journal Blood. An accompanying editorial explains why researchers are optimistic about CPX-351.
Thursday, May 15, 2014
Mayo Clinic early trial uses measles virus to treat blood cancer
 |
| Mayo Clinic YouTube |
CANCER DIGEST – May 15, 2015 – In a first in class clinical trial, Mayo Clinic researchers have demonstrated a new approach to treating blood cancer using an engineered dose of the measles virus. Two patients in the early stage trial with multiple myeloma, a cancer of the plasma cells, were given doses of the virus and both have responded with reductions of cancer in the bone marrow. One patient, a 49-year-old woman, experienced complete remission of myeloma and has been clear of the disease for over six months. The findings appear in the journal Mayo Clinic Proceedings.














No comments:
Post a Comment